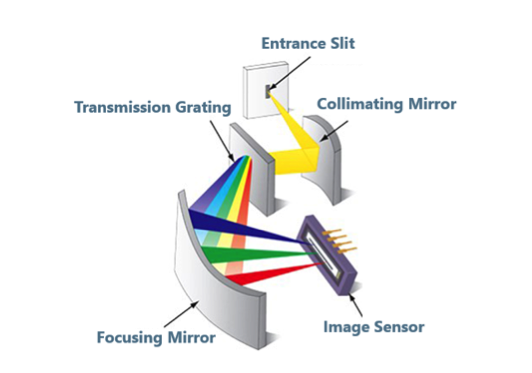
Spectroscopy is an optical analysis technique which measures the different frequency or wavelength components (colors) of a light source and uses this information to determine the physical and chemical properties of the material samples with which the light interacted. Variations in atomic or molecular structure create different spectroscopic “signatures”, so the materials under interrogation can be identified and quantified. The devices used to collect and measure the intensity of each wavelength of light in these signatures are called optical spectrometers.
What is Spectroscopy?
Raman Spectroscopy
A vibrational spectroscopic technique that provides information on material structure, chemical constitution, and dynamic behaviour. It harnesses the one-in-a-million inelastically scattered incident laser photons to reveal these parameters and other critical sample properties.
The far-reaching benefits of this information-rich technique can only be fully unleashed with the highest performance spectrometers.
Our knowledge and expertise within Raman spectroscopy allows us to focus on solutions that provide easy and rapid measurements in a matter of seconds. There are several other types of optical spectroscopy, including Emission, and Absorption.
Emission Spectroscopy
A hot or electrically energized material sample will emit certain specific colors based on the composition of the material. One good example is high-pressure sodium streetlights, which glow a characteristic yellow color due to the presence of sodium atoms. Sending the light from a sodium lamp into an optical spectrometer will show two sharp peaks at 589.0 and 589.6 nm (in the yellow region of the visible spectrum). A mercury vapor streetlight, on the other hand, has strong emission lines at 404.7 and 435.8 nm, creating a characteristic purple glow.
Absorption Spectroscopy
Cooler material in front of a hot continuum source will absorb specific colors from the broadband spectrum based on the composition of the material. A good example is the outer atmosphere of the Sun, which generates absorption lines that can be attributed to individual elements. The same 589.0 and 589.6 nm wavelengths of sodium, for example, are seen as dark gaps (absorption lines) in the solar spectrum due to the presence of cool sodium gas in the Sun’s outer layers.


