Raman spectroscopy has proven to be a technological innovation in the pharmaceutical industry by becoming one of the most popular analytical measurement tools. Coupled with chemometrics methods, it has significant advantages of high sensitivity and resolution in pharmaceutical quantitative analysis. It can be used in the characterization of drug molecules to revealing solid, liquid and solution phase samples arising from polymorphism and quantitative applications in formulations.
Faster, High-Precision Measurement
Real-time process analysis for your pharmaceutical manufacturing needs
Tornado’s HyperFlux™ PRO Plus is currently in use across the globe in both batch and continuous pharmaceutical manufacturing. What’s unique about our technology is that the HyperFlux™ PRO Plus uses a proprietary HTVS™ enabled spectrometer to deliver better Raman sensitivity than ever before possible, designed to provide users with faster measurements (10x to 30x) for better process responsivity, better sensitivity for lower detection limits, and permitting reduction of the laser power without sacrificing performance.
Applications
- API analysis & formulations
- Development (optimization, catalyst selection, process understanding such as kinetics, thermodynamics, excursions)
- Process Monitoring (endpoint, excursions, side products, residual starting material)
- Quantification of a Degradant in an Intact Pharmaceutical Tablet
- Crystallization (Polymorphs and Process)
- Blending
- Amorphous Solid Dispersion (ASD)
- Kinetics
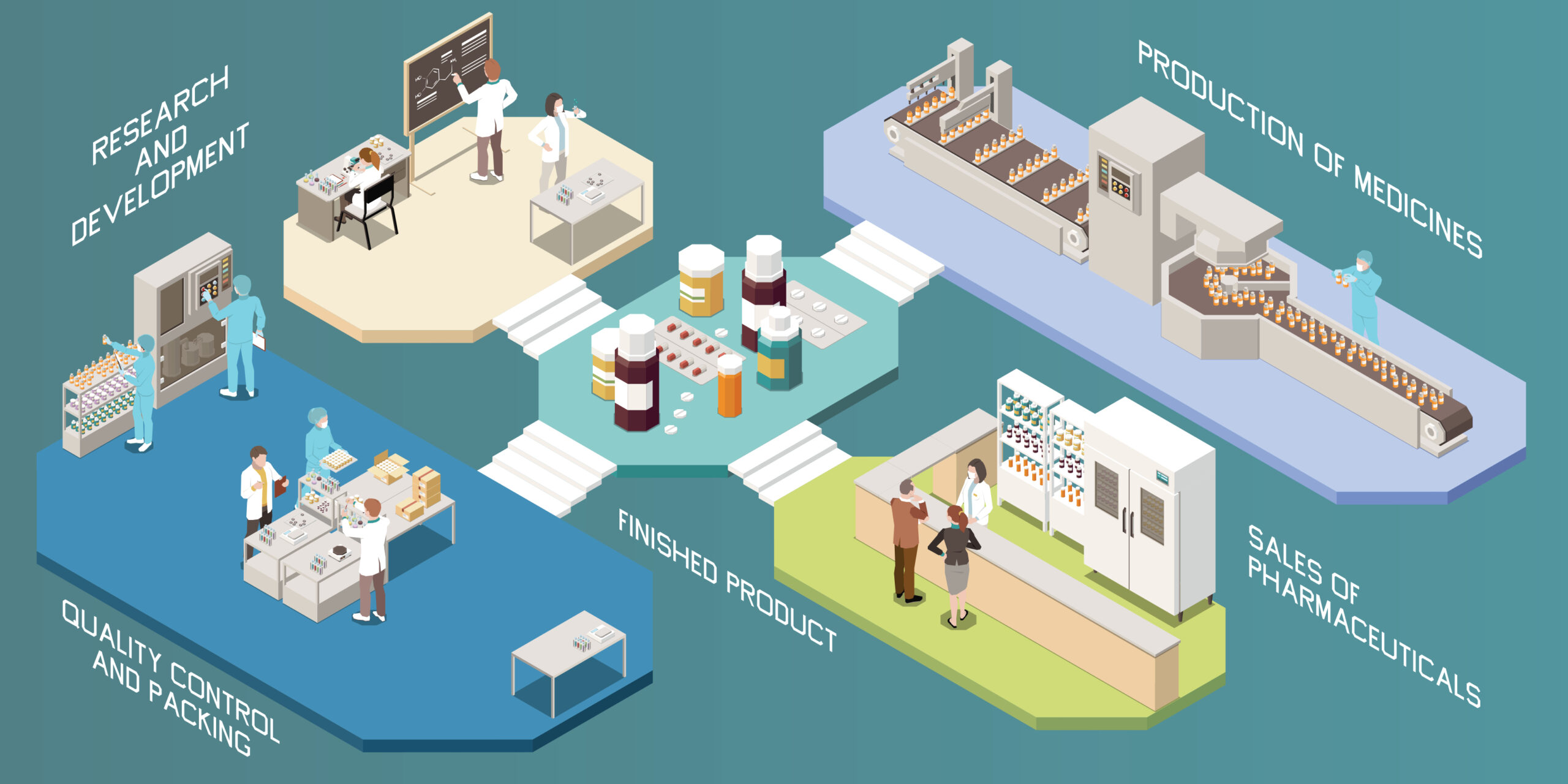
Real-time release strategies can provide a great deal of value to pharmaceutical manufacturing organizations. Benefits can include reduced inventory, reduced laboratory costs, and increased ROI.
Measurement of CQAs and excursions during the Downstream Processing phase of manufacturing can lead to Real-Time Release Testing (RTRT). Real-time evaluation of protein conformational changes, denaturation, aggregation, and other post-translational modifications are among the parameters of interest in the context of RTRT. Detection of these modifications in a Downstream processing scenario would bring significant value and provide impetus toward the goal to create RTRT for protein therapeutic drugs.
Pharmaceutical Related Blogs
“Tornado, a Bruker Company’ Raman solutions enable applications, that were just not doable before.”
What our
clients say
– Sebastian, Analyticon Instruments GMBH